We know that "the thinking brain passes around electrical signals generated by a single class of proteins, the ion channel." (Christopher Miller, 1990). Our main stay in the laboratory is biophysics, specifically a technique called patch-clamp electrophysiology, where we can measure single conformational changes in ion channel proteins that elicit electrical signals, essentially the language of the brain. A form of this approach, called voltage-clamp, was first discovered in the 1950s using a marine animal that possessed very large nerve processes; making it extremely amendable for recording. Today we have access to many types of cells by placing a glass micropipette (electrode) onto the plasma membrane of the nerve cell and creating a tight seal onto a patch of membrane, hence the name of the approach. The electrode is connnected in series to a suite of electronics that is driven by a computer. The signals we are measuring are ~9 orders of magnitude below that of a light bulb (picoampere range) and represent ion flux across the plasma membrane through the lumen of the ion channel. The channels are opened or gated by changes in voltage, synaptic release of neurotransmitters, or gradients of other ions that function in the brain.
We are trying to understand how channels that are gated by voltage and that flux potassium ions (voltage-dependent potassium channels) are altered during nerve regeneration and diabetes. One way we can study potassium channels is to insert (transfect) the dna encoding the ion channel protein into a human cell line (HEK293 cells) so that we can easily manipulate its structure and make predictions as to how this will affect ion channel function when co-expressed with other proteins important in insulin signaling or in pathways regulating neurotrophic factors. Ultimately we want to know how ion channel function is altered or modulated during disease. If we mutate the ion channel dna at important regulatory regions (site-directed mutagenesis) and discover the channel is no longer modulated by insulin or growth factors, then we know that is the molecular target operational during the disease state. Another way in which we can explore the electrical activity of the brain is to do so in an in vitro brain slice configuration. In this manner, we can take a 350 micron slice of a mouse brain, perfuse it with oxygen and physiological saline, and record the electrical communcations across connecting networks or synaptic relationships. We find altered action potential electrical signaling in the olfactory bulb for mice that have been challenged with fatty diets and who are obese or diabetic.
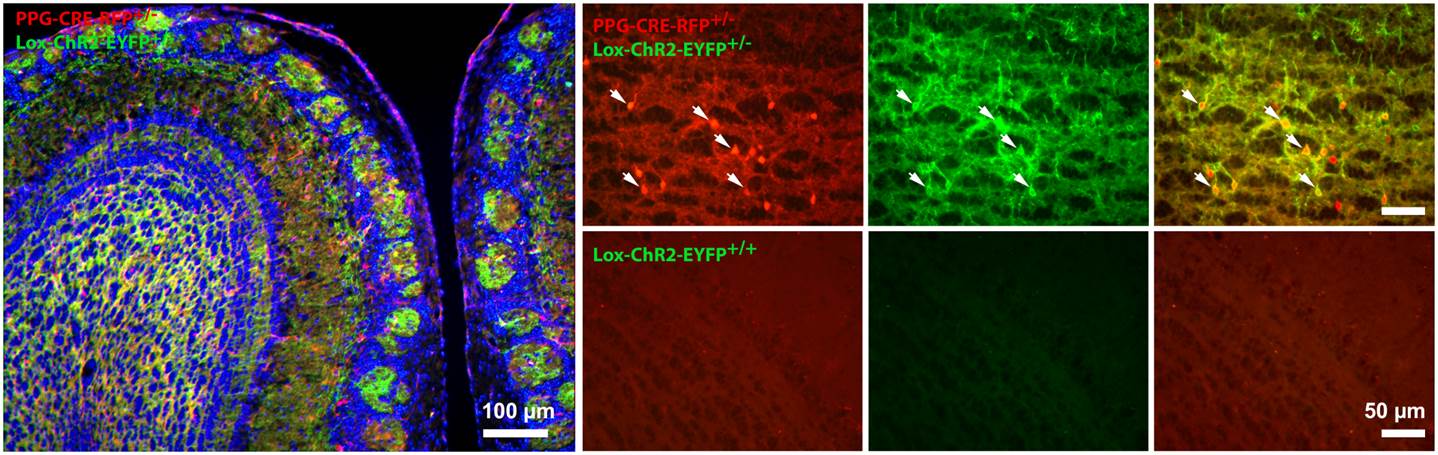
We use the olfactory bulb area of the brain as it has been very well characterized over a century ago by the famous anatomist Ramón y Cajal, and has a very prominent laminar structure so we can be guided to particular neuronal cell types. In order to properly identify the neuronal networks of the affected circuitry in modern times, we can use transgenic mice that contain insertion of genes that code for proteins that are excitable or inhibted by light. This technique, called optogenetics, allows us to stimulate the brain with focused light of a specified wavelength to excite a genetically-identifiable class of neurons to better understand the altered brain physiology of an obese mouse. A final way in which we can explore the activity of ion channels and the neurons they electrical govern is through a technique called electroolfactogram. In this approach, we can deliver an odorant solution through an olfactometer to the entire olfactory organ and then record the collected electrical activity from thousands of neurons simultaneously to understand the health of the entire organ following a disease state or in response to a metabolic factor or hormone.